What Are the Key Differences Between IND and NDA?
In the realm of drug development, two pivotal regulatory submissions to the U.S. Food and Drug Administration (FDA) are the Investigational New Drug (IND) application and the New Drug Application (NDA). Each plays a distinct role in the journey of a new drug from concept to market. Understanding the differences between them is crucial for companies aiming to navigate the regulatory landscape successfully. Here, we delve into the key differences that distinguish an ind vs nda and their significance in the drug development process.
How Do IND and NDA Contribute Differently to Drug Approval Success?
Purpose and Function of the IND
The primary purpose of an IND is to seek authorization from the FDA to begin clinical trials on human subjects. This step is essential after preclinical research has shown promise in animal models. The IND application must demonstrate that the compound is reasonably safe for human exposure and explain how the planned clinical trials will be conducted. It includes details on manufacturing, pharmacology, toxicology, and protocols for studies in humans. An important part of the IND process also involves ensuring that there is sufficient data from laboratory and animal testing to justify moving forward with human trials. The FDA reviews this application to ensure participants’ safety and the scientific quality of the proposed study design.
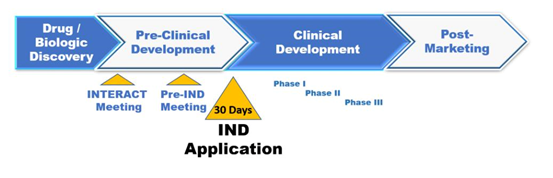
Purpose and Function of the NDA
Conversely, the NDA is submitted once clinical trials (Phases I, II, and III) have sufficiently demonstrated a drug's safety and efficacy. This application seeks FDA approval to market the drug to the public. The NDA is comprehensive, including all data gathered during the animal studies and human clinical trials. It also outlines the drug’s behavior in the body and its chemistry, manufacturing, and controls information. Ultimately, the NDA aims to convince the FDA that the drug is safe and effective for its intended use and that the benefits outweigh any risks. While the IND marks the beginning of human trials, the NDA represents the culmination of the drug's development, summarizing all evidence collected and setting the stage for commercialization.
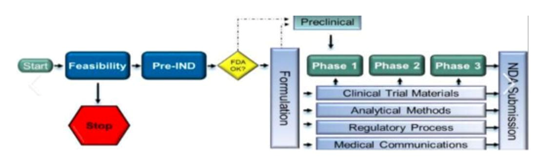
Components and Requirements
The content requirements for INDs and NDAs are distinctly different, reflecting their unique purposes. An IND consists of three main categories of information: Animal Pharmacology and Toxicology Studies, which provide data to justify the initial administration of a drug in humans; Manufacturing Information, which describes the composition, manufacturer, stability, and controls used for manufacturing the drug substance and product; and Clinical Protocols and Investigator Information, which detail the protocols for the proposed clinical studies and information about the qualifications of clinical investigators. For the NDA, the scope is broader. This application comprises a summary of all preclinical and clinical findings, including detailed reports of all clinical trials, statistics, and analyses. It also contains extensive data on the drug’s chemistry, manufacturing processes, labeling, and proposed packaging. Additionally, the NDA includes detailed instructions for use, potential side effects, and other critical information necessary for healthcare professionals to prescribe the drug safely.
Significance in Drug Development
The distinctions between IND and NDA are vital within the context of drug development, highlighting the different stages and objectives involved in bringing a new drug to market. The IND serves as a gateway to human testing, safeguarding participants' safety while enabling researchers to explore therapeutic potential. It sets the stage for systematic investigation through carefully controlled clinical trials, producing data that will later underpin the NDA submission. Meanwhile, the NDA represents the culmination of years of research and development. It embodies a comprehensive package of evidence demonstrating a drug’s therapeutic efficacy and safety profile. Securing NDA approval is the ultimate validation of a development program, allowing a company to market its product and recoup its investment.
Conclusion
Ultimately, both the IND and NDA are indispensable to the rigorous process of drug development, ensuring that only those therapies that meet stringent safety and efficacy criteria reach patients. Their roles reflect the commitment to maintaining public health standards while fostering innovation in medical treatment. Of course, if you find this article helpful, you may wish to share it with your family and friends
